Company
Our vision is for our VivoSight OCT system to become the standard of care for the non-invasive diagnosis and monitoring of certain diseases and conditions that affect cutaneous and epithelial linings of the body.
What we do
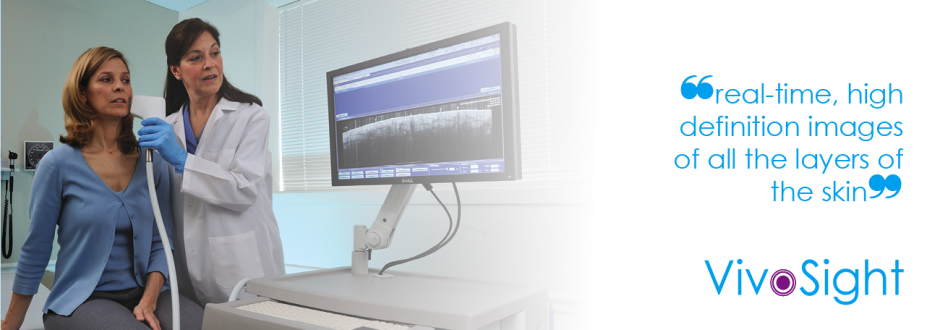
We developed the ‘VivoSight OCT scanner’: a revolutionary device that enables dermatologists to see beneath the surface of skin, to help them make better clinical decisions, faster, more accurately, and without an invasive procedure. It may transform the way dermatologists diagnose and treat common skin conditions such as non-melanoma skin cancer and actinic keratosis. It promises to make as big a change to dermatology as the X-ray made to dentistry!
History
Michelson Diagnostics was founded in 2006 by five experts in laser scanning. In just four years the team developed, built, tested and won regulatory clearance for it in Europe and USA.
In 2011 the VivoSight OCT scanner was commercially launched in Germany, and quickly attracted a core following from renowned Key Opinion Leaders in skin imaging and skin cancer treatment.
Today, OCT imaging with VivoSight is now a regular Dermatology conference topic, more than 50 papers and articles have been published featuring VivoSight images and data. VivoSight scanners are used in five European countries, USA, Australia and Brazil, and over 2,500 patients have been scanned in order to make a clinical decision. OCT is being used routinely in Germany as a viable alternative to diagnostic biopsy of lesions suspicious for basal cell carcinoma, providing real-time, high definition, easy to use and accurate images.
Markets
The market for VivoSight OCT is practising clinical dermatologists (there are at least 25,000 in Europe, USA and Australasia), and also researchers into clinical and commercial dermatology.
Key applications are:
- Non-melanoma skin cancer (NMSC)
- Actinic Keratosis (AK)
- Onychomycosis (Nail fungus)